Sweet Tooth
Bacteria love sugar. In particular, bacteria love glucose, which is easily digestible and quickly converted to chemical energy. When glucose is plentiful, bacteria ignore other nutrients in their environment, feasting on their favored source. But, when glucose is rare, they shift gears and mobilize the machinery needed to use other sources of energy.
Second Messengers
Bacteria use an unusual modification of ATP, the molecule that carries chemical energy in the cell, to notify its synthetic machinery about what it is currently eating. As glucose levels drop, the cell-surface enzyme adenyl cyclase is activated. It grabs ATP molecules, clips off two phosphates, and reconnects the free end back onto the molecule, creating an odd little molecular loop through the phosphate. This product, called cyclic AMP, is released and it spreads through the cell, stimulating production of the enzymes that process other food molecules. Because of its role in delivering messages from the primary glucose sensor (adenyl cyclase) to the synthetic machinery, cyclic AMP is often known as a second messenger.
Reading the Message
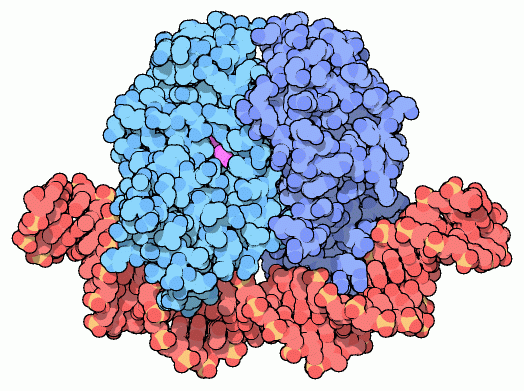
Catabolite activator protein (CAP), also known as cyclic AMP receptor protein (CRP), is activated by cyclic AMP and stimulates synthesis of the enzymes that break down non-glucose food molecules. It is composed of two identical subunits, shown here in blue from PDB entry
1cgp . When cyclic AMP (shown in purple) binds, it changes the conformation of the protein slightly, making it perfect for binding to DNA (shown here in red). CAP binds to a specific DNA sequence, which is found next to the genes that are activated. As shown below, when CAP binds to DNA, it coaxes
RNA polymerase into place, beginning transcription.
Get a Grip
CAP is not gentle when it binds to DNA: it grabs the DNA and bends it by nearly 90 degrees. This is not a smooth bend, however. Since DNA is a double helix with two grooves that spiral up along the strand, it bends more easily in some directions. CAP bends the DNA sharply at two positions where protein alpha helices contact the compressible DNA major groove.

CAP-DNA complex (top) interacting with RNA polymerase (yellow and green, bottom). A flexible linker in the polymerase subunit is not seen in the structures and is shown schematically with dots.
Download high quality TIFF image
CAP in Action
CAP lures RNA polymerase close to the DNA, stimulating the transcription of genes that are nearby. RNA polymerase, shown in yellow from PDB entry
1iw7 , has a subunit that reaches up and interacts with CAP and the DNA. In the RNA polymerase structure, only half of this subunit is seen. PDB entry
1lb2 reveals how the other half binds to both CAP (blue), and DNA (red). The two halves of this subunit are connected through a flexible linker, shown with little yellow dots. Notice that only a small piece of DNA is shown here; inside cells the DNA is part of a long continuous strand. The flexible linker allows RNA polymerase to reach neighboring regions of the DNA and start transcription.
CAP recognizes a specific sequence of DNA, binding only to special sites next to genes involved in energy production. The recognition is provided by interactions between CAP amino acids and DNA bases in the major groove. This picture, from PDB entry
1cgp , shows how two arginines and a glutamate reach over and form specific hydrogen bonds with the DNA bases. Notice also the big bend in the DNA at the thymine-adenine base pair at center. CAP also interacts with many sites on the phosphate backbone of the DNA, strengthening the binding. PDB entry
1cgp has a break in the DNA chain at a key point in the backbone (shown with a star), but you can explore how CAP interacts with this site in PDB entry
1j59 .