Each of your cells contains about 2 meters of
DNA, all folded into the tiny space inside the nucleus, which is a million times smaller. As you might imagine, these long, thin strands can get tangled very easily in the busy environment of the nucleus. To make things even more complicated, DNA is a double helix, which must be unwound to access the genetic information. If you have ever tried to unravel the individual fibers in a piece of rope, you will understand the knotty problems that this can cause. To help with these problems, your cells build several different topoisomerase enzymes that untangle and relax DNA strands.
Relaxing DNA
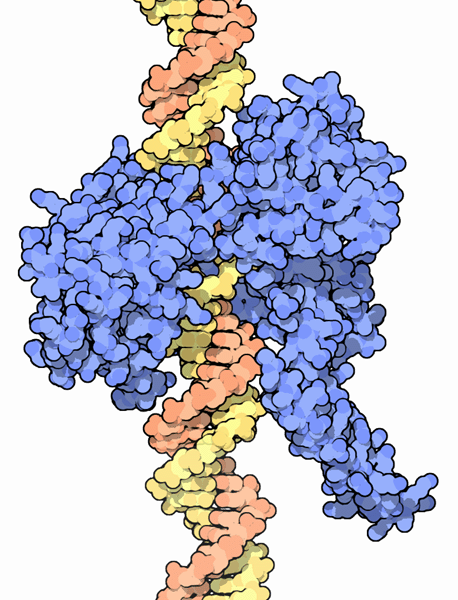
Class I topoisomerases solve the problem of the tension caused during the winding and unwinding of DNA. One example is shown here from PDB entry
1a36 . It wraps around the DNA and makes a cut in one strand. Then, while holding onto the damaged spot, the enzyme allows the helix to spin, releasing any overwinding or underwinding. Once the DNA is relaxed, the topoisomerase reconnects the broken strand, restoring the DNA double helix.
Untangling DNA
Class II topoisomerases, shown on the next page, specialize in untangling DNA in the nucleus. For instance, when a cell is dividing, it needs to separate the two copies of each chromosome. During this process, portions of the two sister chromosomes may become looped around each other, getting hung up together as they are separated. Class II topoisomerase solves this problem by allowing one DNA helix to pass through the other one. It cuts both strands of one DNA double helix, keeping a firm grip on both halves. Then, it passes the other DNA through the gap, resolving the tangle. Finally, it reattaches the broken ends, restoring the DNA.
Toxins and Treatments
These processes of relaxing and untangling are essential for the proper maintenance of your DNA, so topoisomerases are sensitive targets for poisons. If topoisomerases are blocked, the cell will encounter problems during transcription of the DNA and during cell division. Cancer chemotherapy takes advantage of this, using drugs that block topoisomerases to kill rapidly-dividing cancer cells. For instance, the widely-used anthracycline drugs, like doxorubicin and daunorubicin, attack class II topoisomerases, and the plant toxin campothecin blocks the relaxing action of class I topoisomerases.
Class II Topoisomerase
Class II topoisomerase performs the amazing feat of breaking a DNA double helix, passing another helix through the gap, and resealing the double helix behind it. The picture shown here is built from two PDB entries:
1bgw has the lower part of the topoisomerase, and
1ei1 is a domain from a gyrase, which is similar to the upper part of the topoisomerase. The topoisomerase is thought to be a highly dynamic structure, with several gates for entry of DNA into the two DNA-sized holes. Two tyrosine amino acids, shown in red, cleave the DNA strands and form a covalent bond with them, holding them tightly until the DNA can be restored.
PDB entry
1a31 captures a class I topoisomerase in the middle of relaxing a strand of DNA. A tyrosine has broken one strand of the DNA, and formed a covalent bond to the phosphate group at the end of the broken strand. After the strands have spun a few times to release any tension, the enzyme will reform the broken bond in the DNA backbone.
This picture was created with RasMol. You can create similar pictures by clicking on the accession codes here and picking one of the options for 3D viewing.